Structural Analysis of Glycopeptides
Sugar Chain Structure, Saccharide Composition
Structural Analysis of Glycopeptides
Structural Analysis of Glycopeptides in Mouse Monoclonal Antibodies
The major sugar chains in glycopeptides are broadly classified into O-type sugar chains and N-type sugar chains, according to the type of bonded amino acid residue.
The O-type sugar chains are bonded to serine or threonine residue in the protein. The N-type sugar chains are bonded to asparagine residue.
The guidelines require the analysis and data acquisition of the sugar chain bonds in the protein to the maximum extent possible.
This is an example of the structural analysis of glycopeptides in mouse monoclonal antibodies.
Test and research flow
- Use SDS-PAGE to excise bands corresponding to antibodies from the stained gel.
- Perform in-gel digestion with trypsin.
- Separate glycopeptide fractions from peptide fractions using Sepharose* CL-4B (GE Healthcare, USA), and analyze the glycopeptide fractions by mass spectrometry.
- Perform MSn analysis using the AXIMA Resonance.
* Sepharose is a tradename of GE Healthcare.
Results
Fig. 1 shows that the glycopeptides are efficiently recovered in the Sepharose-bonded fractions.
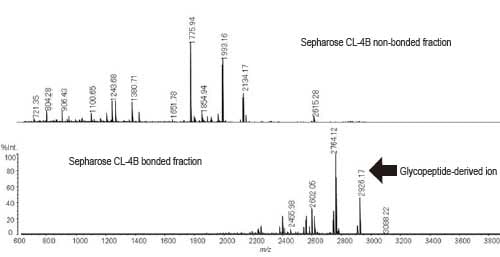
Fig. 1 MS Spectrum of Sepharose CL-4B Fractions
(Positive Liner Mode)
Fig. 2 is the MS/MS spectrum using m/z 2764 as the precursor ion (monoisotopic peak, equivalent to m/z 2767 molecular ion in Fig. 1).
Ions with sugar fully desorbed and peptide ions were observed.
Fig. 3 shows the MS/MS/MS spectra of the ion with sugar fully desorbed (m/z 1157) and the pre-desorption ion (m/z 1240). Comparison of these spectra reveals the glycopeptide sequence to be "Glu-Glu-Gln-Phe-Asn-Ser-Thr-Phe-Arg" and the sugar chain-bonded amino acid residue to be asparagine (Asn).
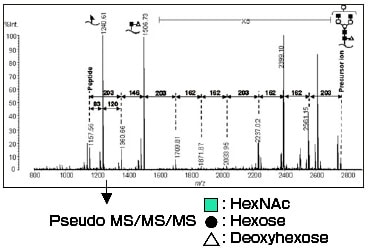
Fig. 2 MS/MS Spectrum (m/z 2764, AXIMA-QIT)
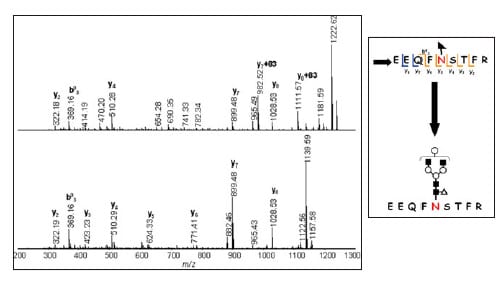
Fig. 3 MS/MS/MS Spectrum (top, m/z 1240, AXIMA Resonance) Pseudo MS/MS/MS Spectrum (bottom, m/z 1157, AXIMA Resonance)


