Accelerating the pre-clinical/clinical phase
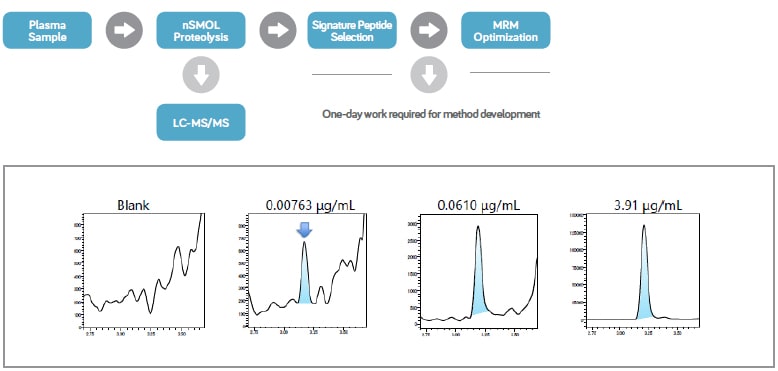
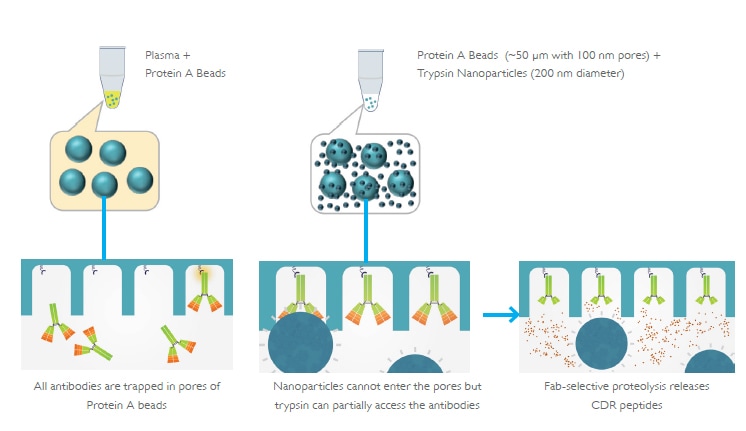
Bioanalytical method development is the critical step in the biopharmaceutical pipeline as it bridges the transition to pre-clinical and clinical phases. Ligand-binding assay (LBA) has been the common technique for biologics, however, LC-MS is emerging as an alternative to reduce time and cost needed for method development and to gain increased selectivity and efficiency. To further simplify and streamline the LCMS workflow for antibody bioanalysis, Shimadzu developed an innovative nanotechnologybased nSMOL™ Antibody Bioanalysis platform for the selective proteolysis of the Fab region of antibody drugs. This increases the detection sensitivity of surrogate peptides in CDR regions, which can be accurately quantified via MRM measurements using a triple quadrupole high performance liquid chromatograph mass spectrometer.
nSMOL workflow
nSMOL Antibody BA Kit
Simplifying workflows and enhancing sensitivity

| nSMOL + LCMS | LBA | |
|---|---|---|
| Ab for collection/detection | Not needed | 6++ months to develop |
| Cross reactivity test | Not needed | Mandatory and tricky |
| Pre-validation | 1-3 days | 2-3 weeks |
| Full validation | 3-4 w | 3-4 w |
| Sample prep | 3-5 h | 2-4 h |
| Data features | Highly selective and reliable, wide dynamic range, easy to multiplex, independent of antibodies |
Highly dependent on quality of detection Ab. |
Development of LCMS bioanalysis in combination with nSMOL proteolysis is much faster, and can dramatically accelerate the total R&D period of biologics by alleviating the bottlenecks that typically occur when entering the preclinical and clinical phase.